Pitt Researchers Work to Develop and Test Vaccines
August 7th, 2020
Infectious diseases remain a leading cause of illness and death in the United States and around the world. Although some infectious diseases are preventable with vaccines, many others still are not. Emerging diseases such as COVID-19 present new threats. To take on these challenges, the Center for Vaccine Research at the University of Pittsburgh was established to bring together researchers from many different fields at Pitt and UPMC in an effort to find better ways to protect adults and children from life-threatening infectious diseases.
As healthcare providers and scientists work together to develop new vaccines, research participants are needed to take part in clinical trials to test them. To help connect adult and pediatric participants with study opportunities, researchers created the Pittsburgh Vaccine Trials Unit Registry, which is a database of people who volunteer to provide information about themselves and agree to be contacted about vaccine-related clinical trials. Recent studies include a national COVID-19 vaccine clinical trial for people who are at high risk for contracting the disease or who are at high risk for developing severe illness. Future projects may include additional COVID-19 vaccine studies, influenza vaccine studies, and vaccines for certain life-threatening diseases in children.
Speaking about COVID-19 vaccine research during a recent press conference, Judy Martin, MD, co-director of the Pittsburgh Vaccine Trials Unit and pediatric infectious disease specialist at UPMC Children’s Hospital of Pittsburgh, noted that Pitt has long been recognized for its vaccine expertise—in part because the polio vaccine was developed at the university more than half a century ago. “Pittsburgh really has a long history of vaccine research, starting in the 1950s with Jonas Salk,” said Dr. Martin, “I think now with this first [Phase 3 COVID-19] study and the others that may follow, Western Pennsylvania has another chance to participate in these research studies, which are really going to make a difference both in our local community, as well as nationwide and across the world.”
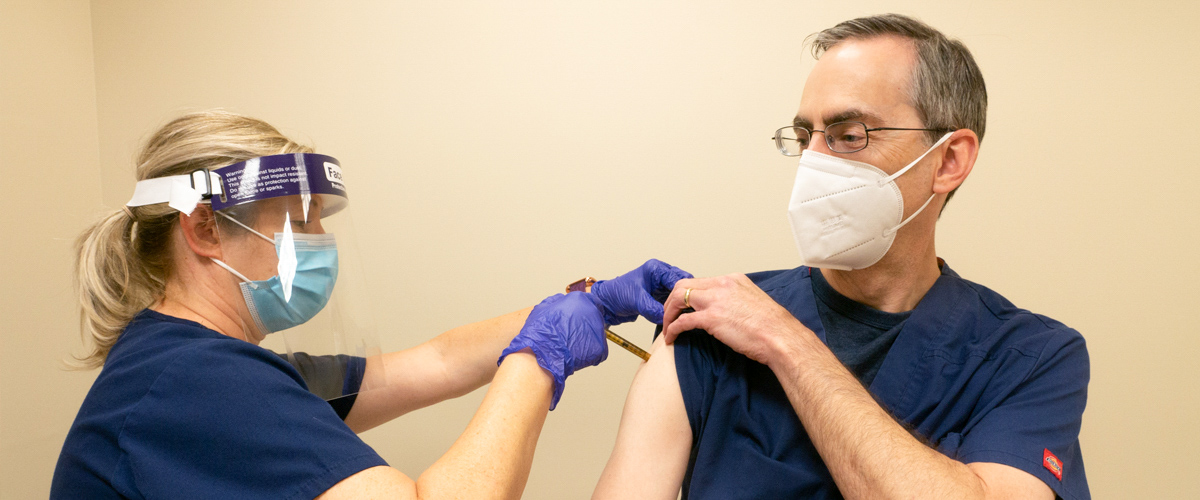
For Steve Reis, MD (pictured above receiving an investigational COVID-19 vaccine), the decision to take part in a vaccine clinical trial was an easy one. According to Dr. Reis, “Vaccines save lives. I am doing this for my family, my patients, and my friends. I am doing this for everyone.”
Interested in vaccine research? Learn more about the Pittsburgh Vaccine Trials Unit Registry for adults and children on the Pitt+Me website, or contact us at PittPlusMe@pitt.edu or 1-866-438-8230.